PO Box 823 Dunedin New Zealand
TOREA TECHNOLOGIES IS ENGAGED IN RESEARCH, DESIGN AND DEVELOPMENT OF BIOTECHNOLOGY DERIVED ANTIBODY PHARMACEUTICALS
PRODUCTS ARE CURRENTLY DEVELOPED FOR CANCER CARE APPLICATIONS
CYTOTOXICS ADC
IMAGING RADIOPHARMACEUTICALS
MRI CONTRAST AGENTS
RADIOTHERAPY
IMMUNOCONJUGATE
PHARMACEUTICALS




CLINICAL APPLICATIONS IN
ONCOLOGY-CANCER THERAPY AND IMAGING
-IMPROVEMENTS
IN TARGETING OF
BIOTECHNOLOGY DERIVED
MONOCLONAL ANTIBODY BASED AGENTS
BIOSELECTIVITY CHARACTERISTICS
PURITY, POTENCY, IMMUNOCOGNIZANCE, BINDING, AFFINITY, PERSISTENCE
Delivery augmentation by bioselective
immunoconjugates of cytotoxics,mri and nuclear medicine agents
Higher tumor uptake,reduction of side
effects and adverse delivery

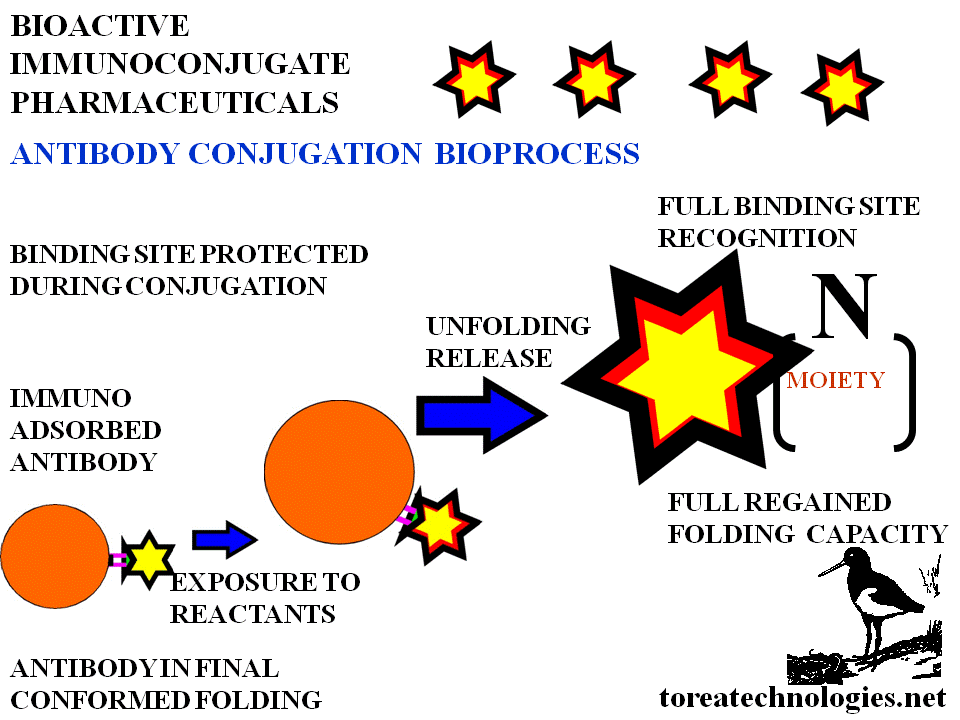
BIOSELECTIVE
IMMUNOREACTIVITY IMMUNOCONJUGATE
DESIGN PREPARATION
-High specific modification whilst
remaining immunoreactive. Binding site protection allows for application
of conjugation chemistry. Bound antibody is able to withstand greater
concentrations of reactants for higher specific modification.Conjugation
applied to the antibody in final bound conformation.
-Bioprocess preparation to allow folding
competency.Folding changes upon binding accomodated with this product.
-Preparitive affinity purification with
epitope based solid phase immunoadsorbent column bioprocess for
production.
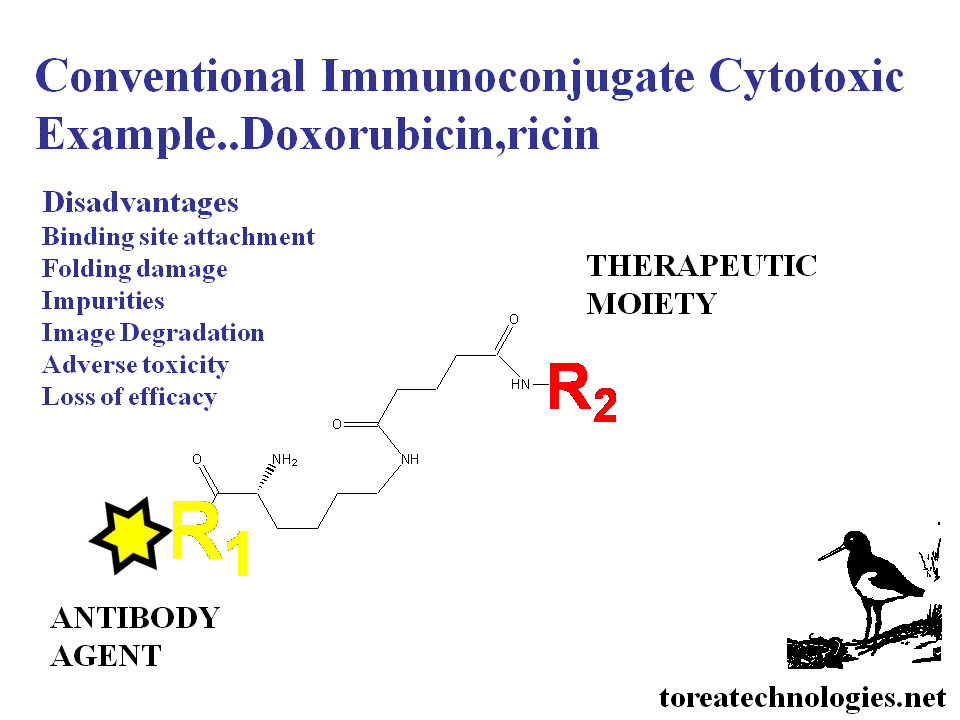
CONTEMPORARY ANTIBODY CONJUGATION LIMITATIONS
Poor targeting,reduced immunoreactivity,low potency,low solubility,damaged folding properties....impurity loss of specificity and of limited clinical value.
The antibody is relatively inert chemically requiring high stoichiometric reactant concentrations with subsequent damage for conjugation.The folded state of free native antibody is metastable to ensure solubility,presentation of the binding site and binding affinity. Folding changes occur with binding as demonstrated with thermodynamic determination and X-ray crystallography studies.
Clinical limitations 1.Low targeting
2.Reduced binding 3.Low potencytocy 4.High background 5.Corrupted
biodistribution
NATURE OF LABELING DAMAGE-IMPURITY
Labeling of the sensitive binding site where intimate contact is required.
Reactant chemical damage
Folding distortion particularly tertiary structure
Low retention and persistence upon binding to antigenic target
Low specific activity
Low solubility-particularly when higher substitution is attempted.
................................................................................................................
MARKERS OF INTEREST
Cancer Pathologies....antigens and
epitopes
![]()
Lung....calcitonin,bombesin,cea etc
Prostate....psa, p-specific membrane antigen
Others....Ovary,melanoma,breast,leukaemia,colorectal....
Non cancer pathologies... Hiv....components of replication....
infection....bacterial viral
Epitope analysis required for refined specificity.
Cancer markers offer a therapeutic window via antigen density and relative specificity.
Epitope mapping of antigens has refined specificity eg TNF epitopes
Growth factor receptors eg EGFR,Cell surface antigens eg glycoproteins and secretory products such as hormones eg calcitonin,oncofoetal antigens eg CEA.
Availability
from biotechnology sources ......
culture,fermentation,peptide,carbohydrate and glycosynthesis.
![]()
![]()
ANTIBODY AGENTS OF INTEREST
SOURCE-Biotechnology,fermentation,culture,monoclonals
FORMS-fragments,single
chain,recombinant derivatives....phage libraries 
...............................................................................
CONJUGATION BIOSELECTIVITY
PROCESS
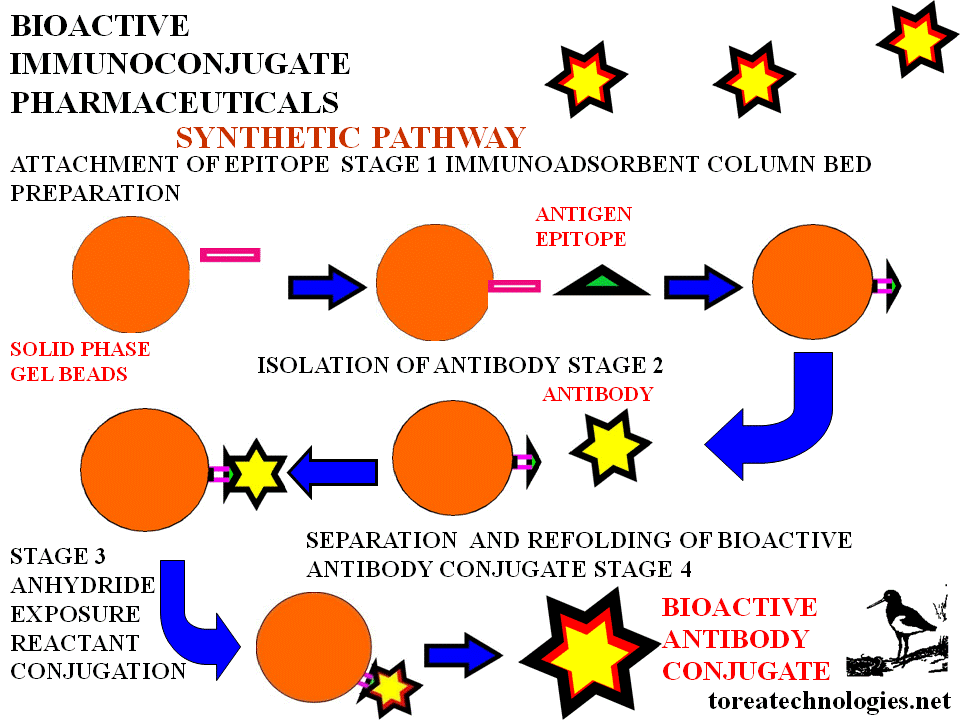
Bioselective antibody conjugation is
effected via a custom made biological substrate (epitope column)
corresponding to the antibody recognition properties.This solid phase with
biologically attached antibody forms a perfused column and a bioprocess
based synthesis, separation and purification system.The bioselective
antibody preparation product is derived from a managed conjugation
chemistry utilizing biological specificity.
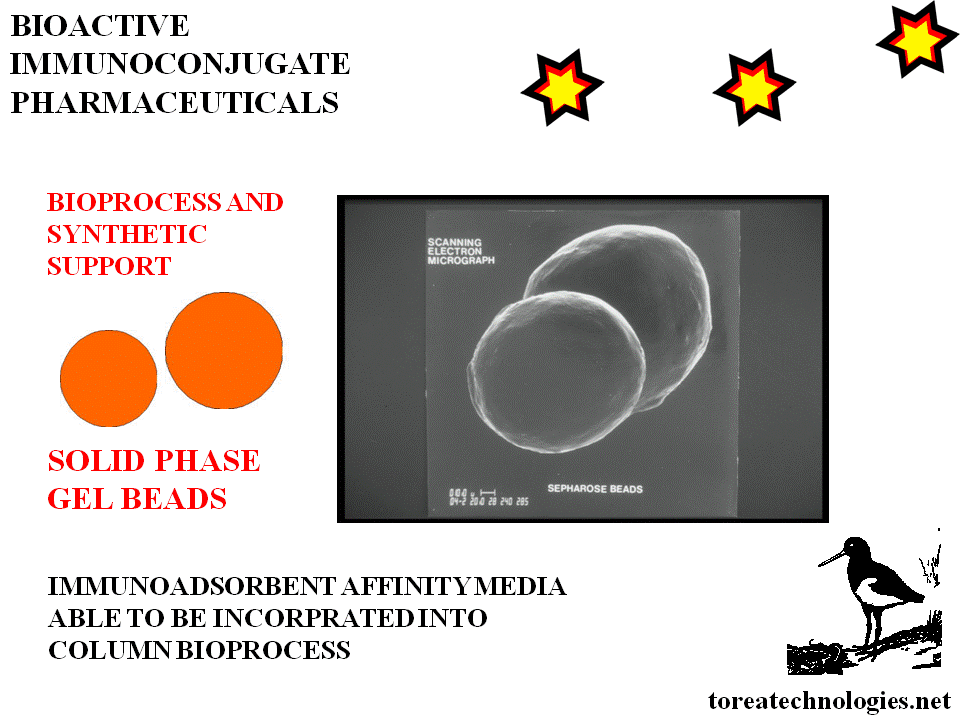
BIOSELECTIVE SOLID PHASE IMMUNOCONJUGATE SYNTHESIS
Custom biological substrate components:
Epitopes-biotechnology,peptide and glycoprotein synthesis.
Attachment to solid phase media usually in bead form for epitope column- sequence of fluidic reagent perfusion to prepare solid phase.
Incubate with antibody fragment,separate wash and purify.
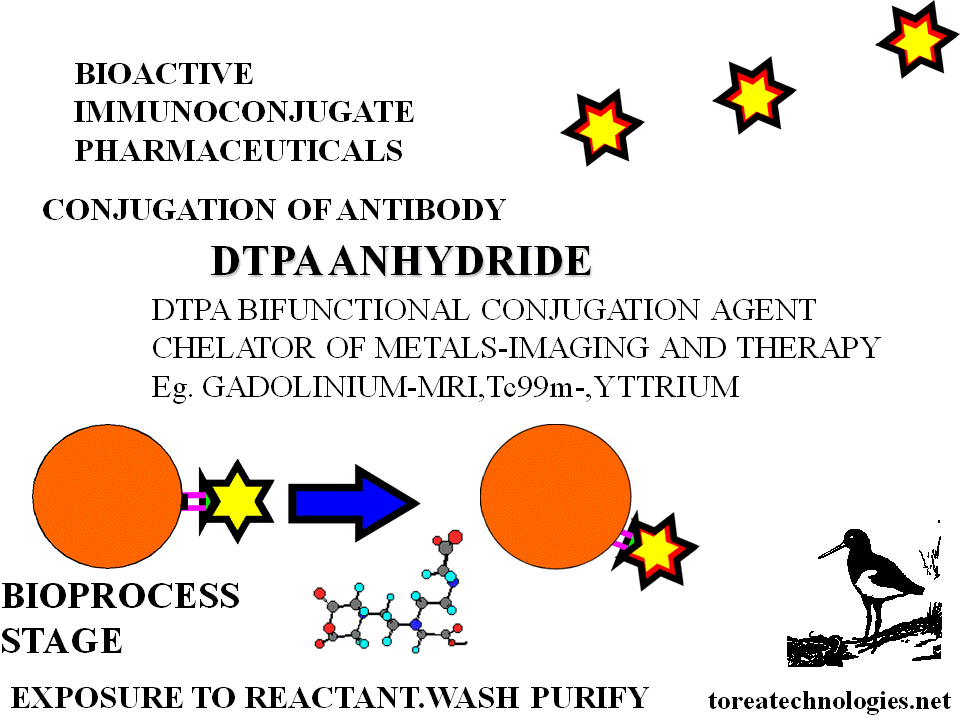
Expose to retroactivated linker reactant (eg.DTPA anhydride). Wash and purify.Chromatographic monitoring in tandem.
Solid phase media with biologically activated matrix allows automation and computer controlled conjugation bioprocess.
NATURE OF LABELING DAMAGE-IMPURITY
Labeling of the sensitive binding site where intimate contact is required.
Reactant chemical damage,excess and byproducts
Folding distortion particularly tertiary structure
Low retention and persistence upon binding to antigenic target
Low specific activity
Low solubility-particularly when higher substitution is attempted.
Clinical limitations
1.Low targeting
2.Reduced binding
3.Low potency
4.High background
5.Corrupted biodistribution
6.Side effects
7.Adverse toxicity
................................................................................................................
CONJUGATION BIOSELECTIVITY
PROCESS
Bioselective antibody conjugation is
effected via a custom made biological substrate (epitope column)
corresponding to the antibody recognition properties.This solid phase with
biologically attached antibody forms a perfused column and a bioprocess
based synthesis,separation and purification system.The bioselective
antibody preparation product is derived from a managed conjugation
chemistry utilizing biological specificity.
BIOSELECTIVE SOLID PHASE IMMUNOCONJUGATE SYNTHESIS
Custom biological substrate components:
Epitopes-biotechnology,peptide and
glycoprotein synthesis.Attachment to solid phase media usually in bead
form for epitope column- sequence of fluidic reagent perfusion to prepare
solid phase. Incubate with antibody fragment,separate wash and
purify.Expose to retroactivated linker reactant
(eg.DTPA anhydride).Wash and
purify.Chromatographic monitoring in tandem.
Solid phase media with biologically activated matrix allows automation and computer controlled conjugation bioprocess.
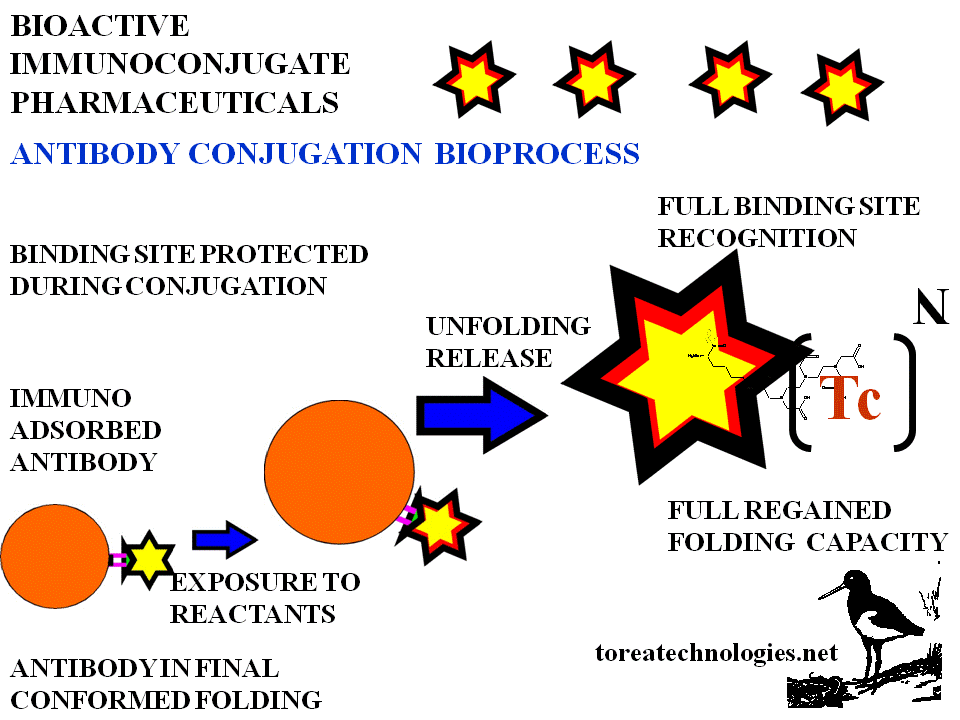
BIOSELECTIVE CONJUGATION MECHANISM
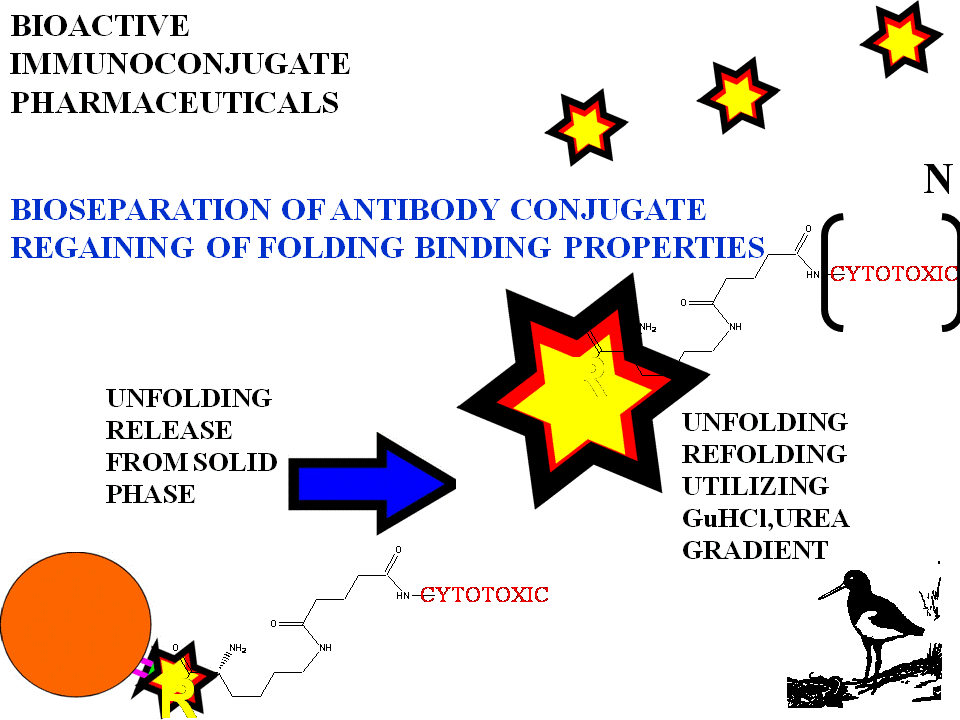
Bound antibody presents a selected conformed area for reactant access.This area is other than the binding site of the antibody.The antibody bound to the antigen has a different conformation to that of the free antibody as folding changes occur with binding.The intimate binding site molecular contact is such that every water molecule is squeezed out. Conjugation is prevented on the binding site being protected by the bound epitope. After reactant contact and washing the antibody conjugate is cleaved and separated from the solid phase by unfolding back into the aqueous phase with subsequent dose and formulation for dispensing. Conjugation reactant chemistry can be with direct or indirect linking agents.
ISOLATION OF ANTIBODY
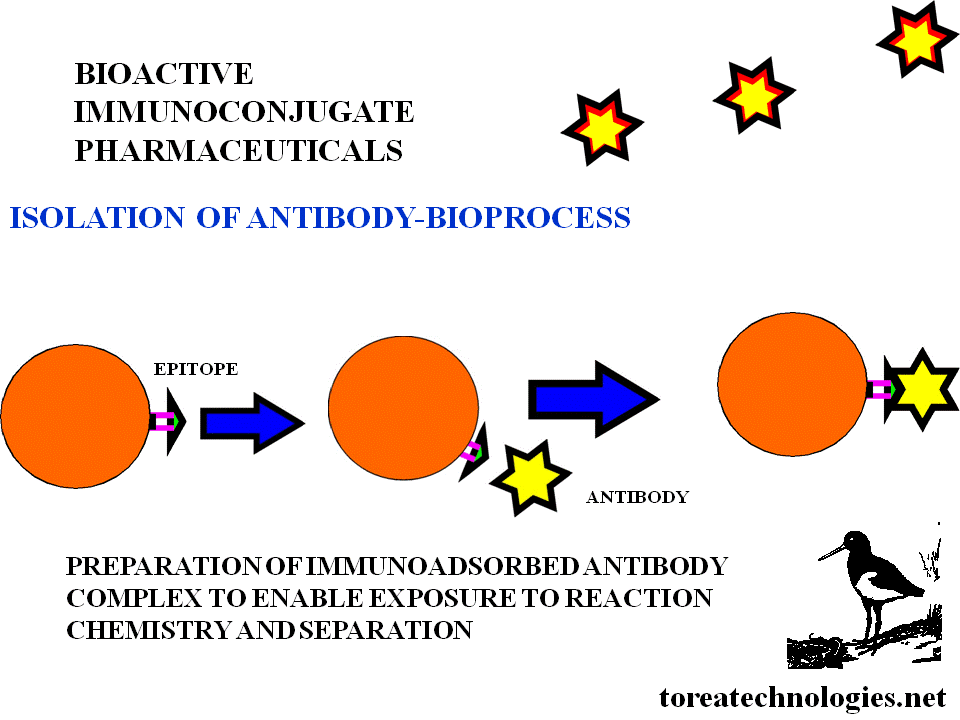
ANTIBODY CONJUGATION
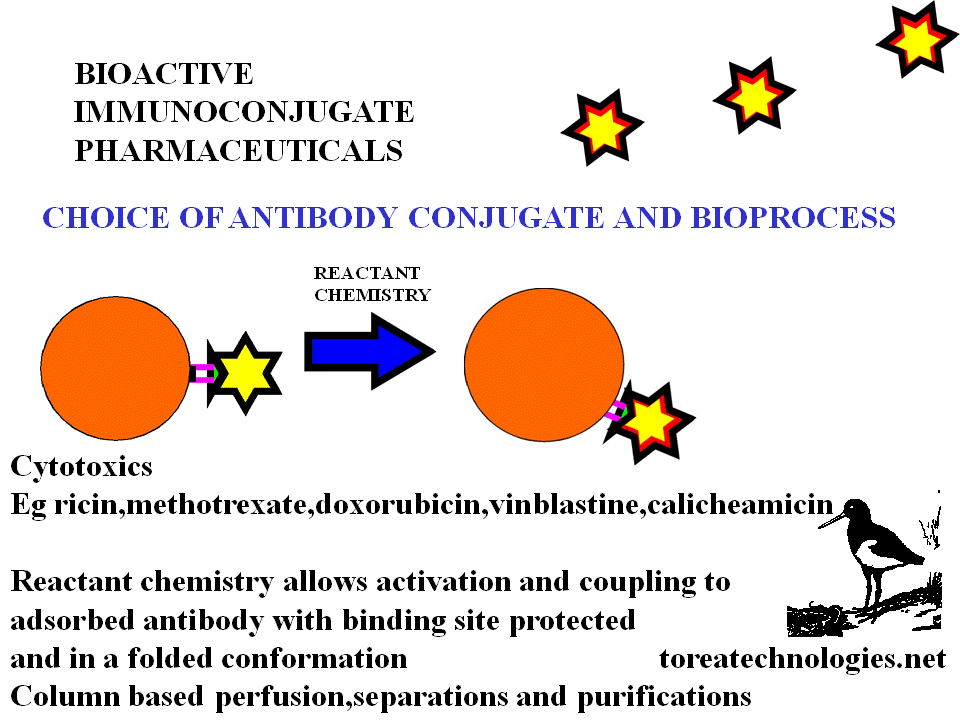
BIOSELECTIVE FOLDING
IMMUNOREACTIVITY
It is well known that antigen binding
fragments of antibodies can be refolded from denatured states with
recovery of their specific binding. Renaturation and refolding is utilized
extensively in separation and purification by biotechnology in product
recovery from cell cultures.Guanidine and urea have been shown to
reversibly unfold-refold in a highly co-operative process and their
folding profiles with urea gradient concentration can be obtained.
Unfolding release of immunoconjugate can be with low concs of denaturant.
An immunoconjugate with folding properties results. This contributes to
supramolecular and dynamic binding properties to enhance targeting ...in
part the folding changes observed with binding. Binding is strong and
persistent.
REFERENCES
1.Journal of Nuclear Medicine 6 27 P1040 1986
2.US Patent No 5,082,928
3.Advances in Radiopharmacology ISBN 0 642 59902 5 Int Symposium on Radiopharmacology (6th:1989 Sydney,Australia)
4.Journal of Nuclear Medicine 32 116-22 1991
5.Journal of Immunological Methods, 133 1990 159-167
LUNG CANCER
Using Nebulized Biotechnology Derived Tc99m Labeled Antibodies For Early Detection Imaging
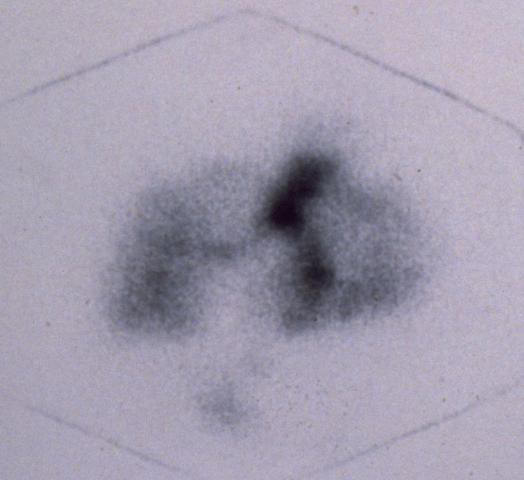
http://users.netaccess.co.nz/lung-cancer
CLINICAL UTILIZATION
Mucociliary Clearance 4hrs
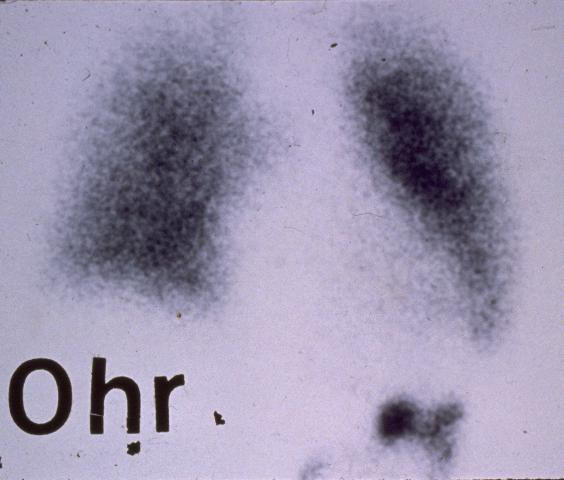
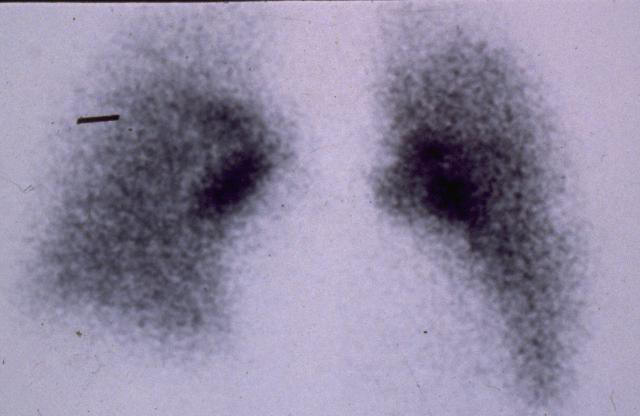
Lung cancer
pathology
Lung cancer is the leading form of cancer death in the US. In 1992 there
were about 168,000 new cases of the disease diagnosed and approximately
146,000 deaths attributed to it. This number is increasing and many cases
are not diagnosed until fairly advanced due to the rapid rate of tumor
growth,soft tissue differentiation identification difficulties with
cytology sampling, radiology and deep fine structure location in the
bronchial tree. Location of cancers occur frequently at bronchial tree
bifurcation points with aerodynamic interception of inhaled carcinogenic
particulates such as tar from cigarette smoke.
Such is the burden on health care resources that tobacco litigation
settlements resulted in $230 billion recently.
Current lung cancer
detection
For the patient the problem has been to detect and diagnose malignant from
harmless lesions and in the US 40,000 resultant invasive exploratory
thoracic surgical operations(thoracotomies) requiring risky full theatre
resection workup and recovery with these investigations are found to be
inappropriate for them. In these cases the bronchial wall lesions can be
benign resulting from other etiology e.g. inflammation,infection and
fibrosis or part of unresectable disease and disseminated. Other options
for inoperable disease that has spread include chemotherapy and palliative
measures.
New imaging technology
A new imaging technology has been developed in Dunedin New Zealand to
photograph epithelial lung cancer by inhalation delivery and localization
of a biospecific contrast tracer. The procedure is simple and
non-invasive involving breathing of a mist and offers imaging with cancer
for early and more specific detailed photography available at hospital
imaging centres on an outpatient basis using currently available ethically
approved clinical materials and procedures. The inhaled biospecific
contrast agent is able to recognize epithelial lung cancer and localize by
antibody reaction carrying the imaging agent with it once inside the lungs
and deposited on the lung epithelium. The resultant images can be used in
treatment planning eg radiotherapy targeting from early diagnosis and
screening.
Inhalation of the biospecific mist is an efficient direct delivery
means achieved with nebulizer produced aerosol having microfine droplet
dimensions to achieve deep lung penetration. Micromist droplet size must
be less than 3 microns. Droplets with greater diameter deposit in the
upper airways(eg throat) with participation in air filtration for
breathing. Coating of the considerable surface of the bronchial tree
occurs beyond the range of the bronchoscope in depth and spatially within
airways architecture.
Biospecificity of
monoclonal antibodies
Differentiating malignant from innocent lesions is achieved by the
monoclonal antibody based tracer which has fine tuned recognition
properties based on a molecular level. Antibodies are utilized in the body
as a protection and recognition means e.g. flus and immunity and have
offered optimism in oncology since their discovery and Nobel Prize winning
biotechnological development. Application of these is widespread and
cytology of tumor tissue identification in sputum and biopsy samples is
used extensively in bioseparation technologies. Monoclonals and
recognition derivatives are now available as pharmaceutical products with
high purity and in quantity from the biotech industry and stock market
investment. Labeling and imaging utilizes the clinical tracer Technetium
used extensively in hospital nuclear medicine clinics and nebulizer
produced aerosol for routine ventilation studies .
Epithelial lung cancers have tumor specific characteristics and the antibodies selected are able to recognize these and immunoreact. Contrast enhancement then occurs with mucociliary clearance sweeping out the unreacted agent from the lung fields and background subtraction for the image process. This natural clearance develops biologically based imaging photographs showing location and extent of the cancer. Mucociliary clearance in participation with lung fine structure acts as a powerful filtration mechanism for our inhaled air.
CHEMOTHERAPY
Cytotoxic Therapy Augmentation with bioselective immunoconjugates
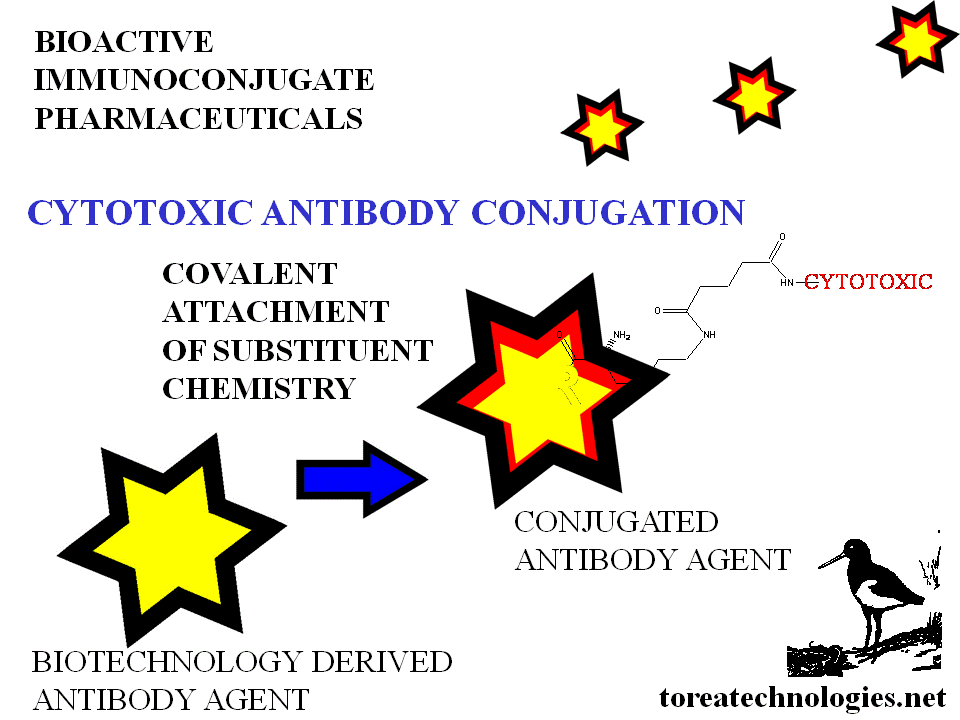
Cytotoxics with more specifically targeted properties- therapeutic index increased with biospecificity.
Useful particularly in cases of motile and diffuse cancers such as leukaemia and occult metastatic deposition.
Increase of dosage-Tolerance of a higher dosage of cytotoxic to be administered with greater concentration at tumor sites increasing oncological kill.
Reduction of side effects-Cytotoxic side effects of current chemotherapy severe-Often from non specificity,cross reactivity and compromised biodistribution. Frequently limits treatment plan eg nausea,wbc and haematological,hair loss,cardiotoxicity and myelosuppression side effects.
CYTOTOXICS OF INTEREST
Bioselective immunoconjugate cytotoxic composites eg methotrexate,doxorubicin
Established mode of action-Antimetabolite,alkylating etc
Widely used,..extensive clinical preparations of source available to pharmaceutical quality.
Known biodistribution,dosage and antineoplastic mode of action.
Toxicological data from
pharmacological and pharmacokinetic studies.
BIOSELECTIVE CYTOTOXIC LINKAGE CHEMISTRY
DIRECT....methotrexate anhydride,activated esters,retroactivated moieties.
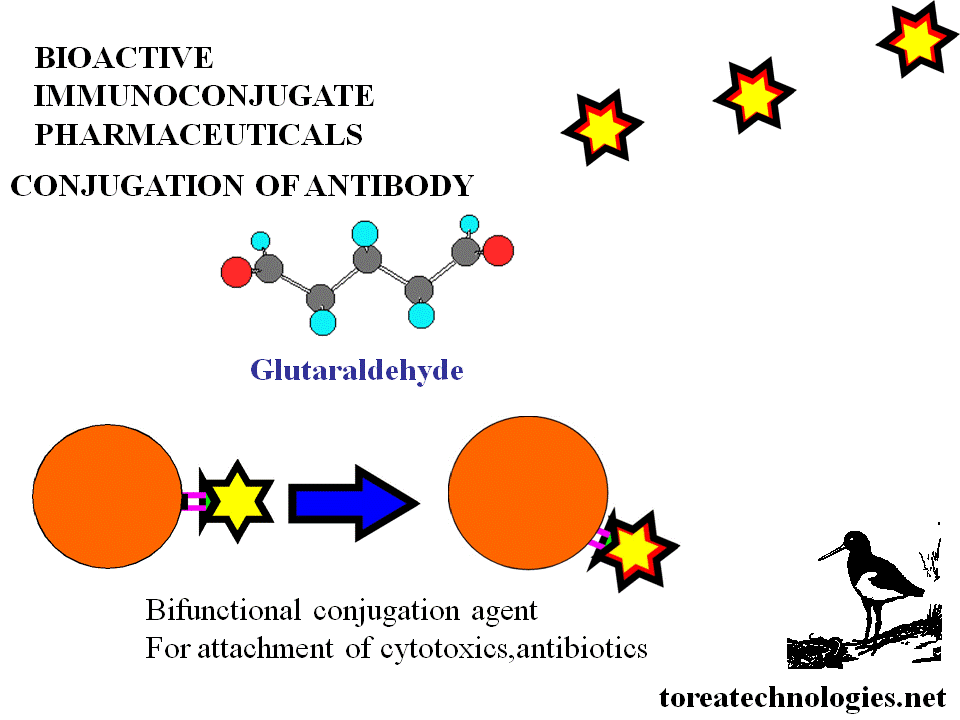
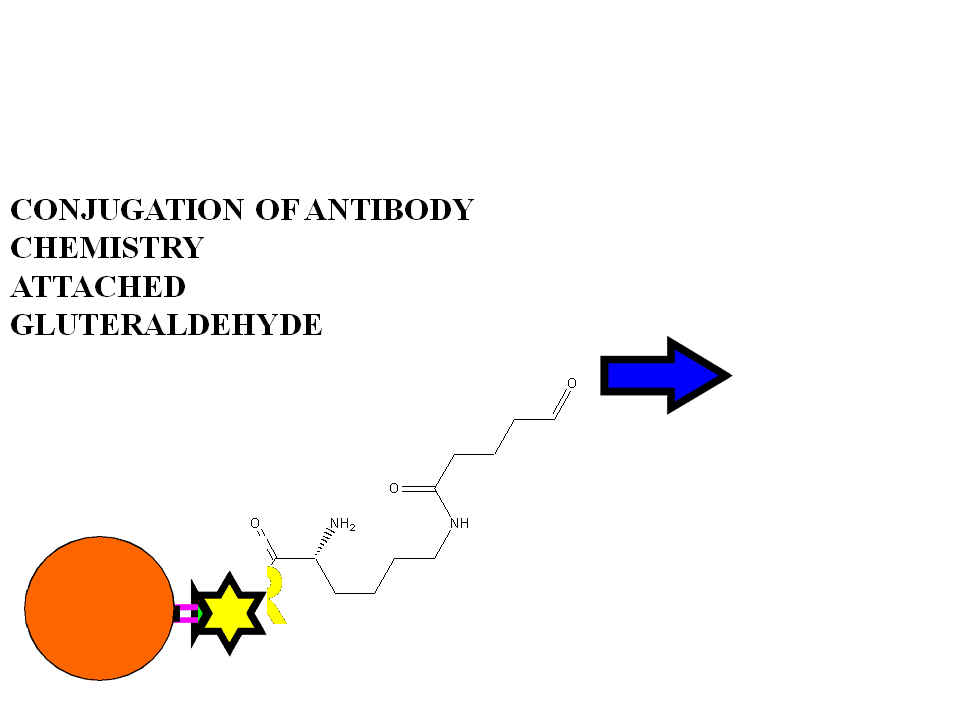
INDIRECT....Linkers..sdtp,glutaraldehyde
Delivery Toxins.... ricin,gelonins,tricosanthins,tricokerin,pokeweed,diptheria and pseudomonas toxins.Cytokines eg interleukin 2
BIOSELECTIVE CYTOTOXIC PRODUCTION
Preparitive Conjugation Bioprocess
Biotech Compatible
Hardware..columns,valves,conduit and flow circuits eg Pharmacia,Biorad
Tandem chromatographic support eg detection and analysis equipment...uv spectroscopy
Reaction and sequence if automated...liquids,reagents,origins and batch
Software archive..script design eg biopilot...commercially supplied
Site...cleanroom suite
Records available for
independent regulatory inspections. Batch requirements...Standard
compliance validation...audit trail.
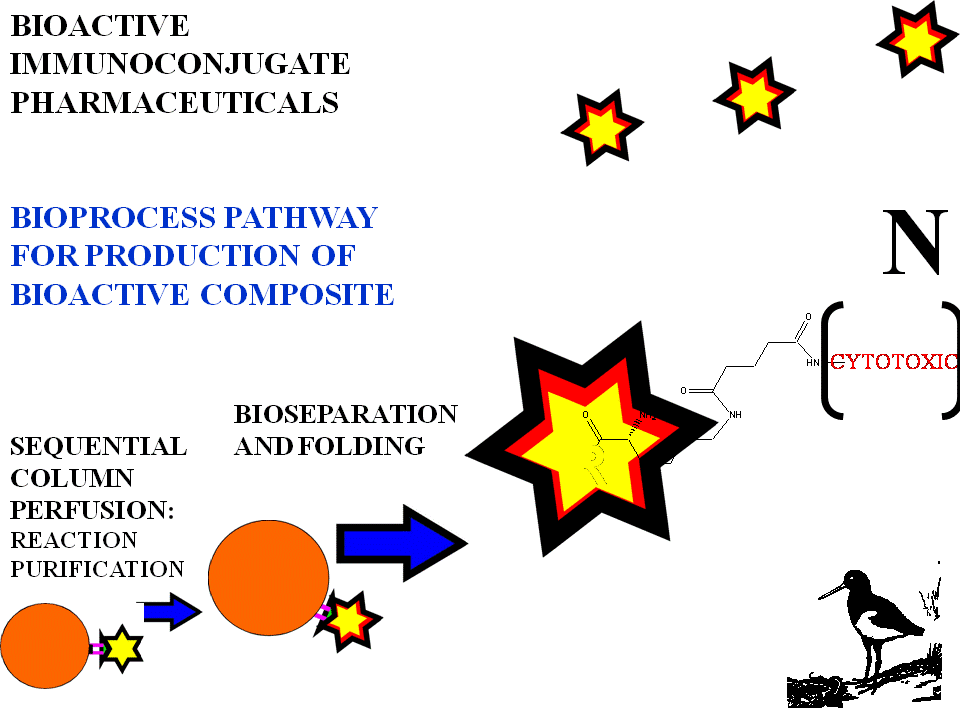
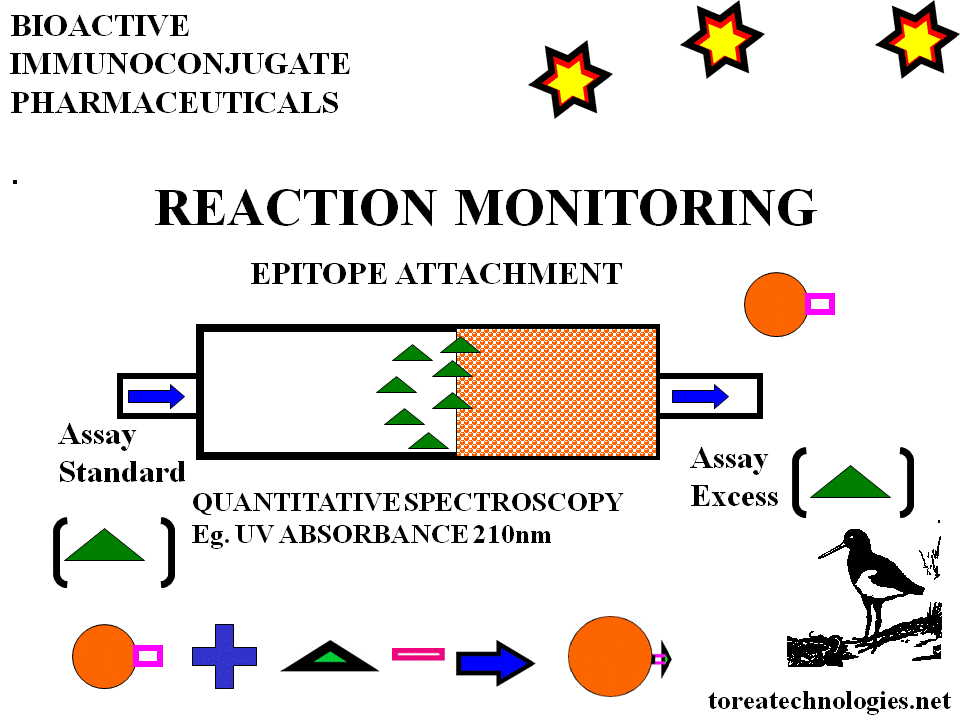
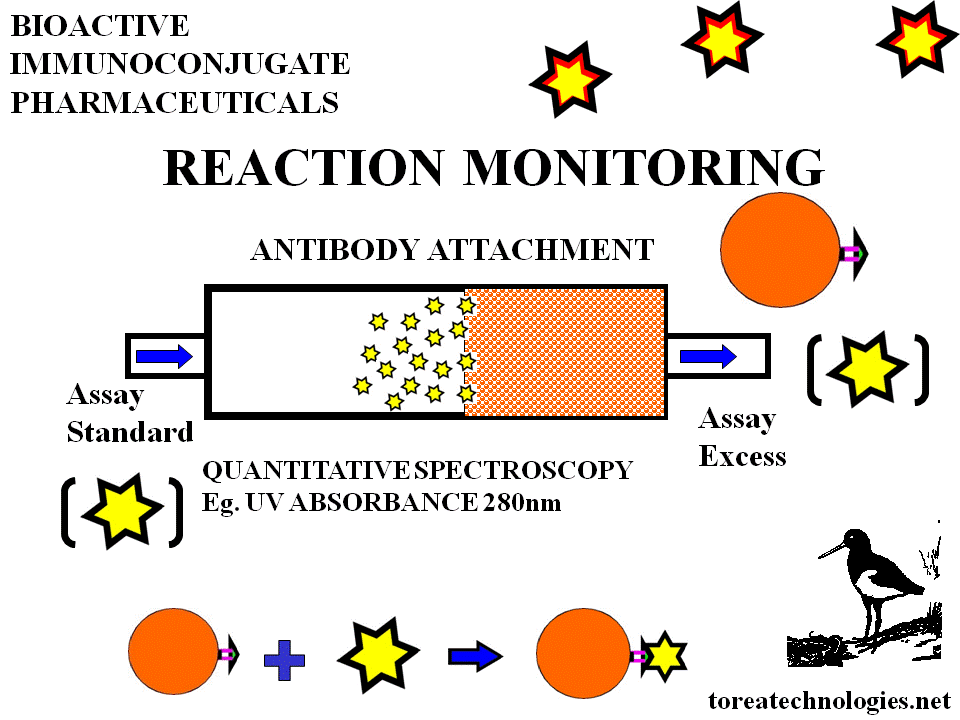
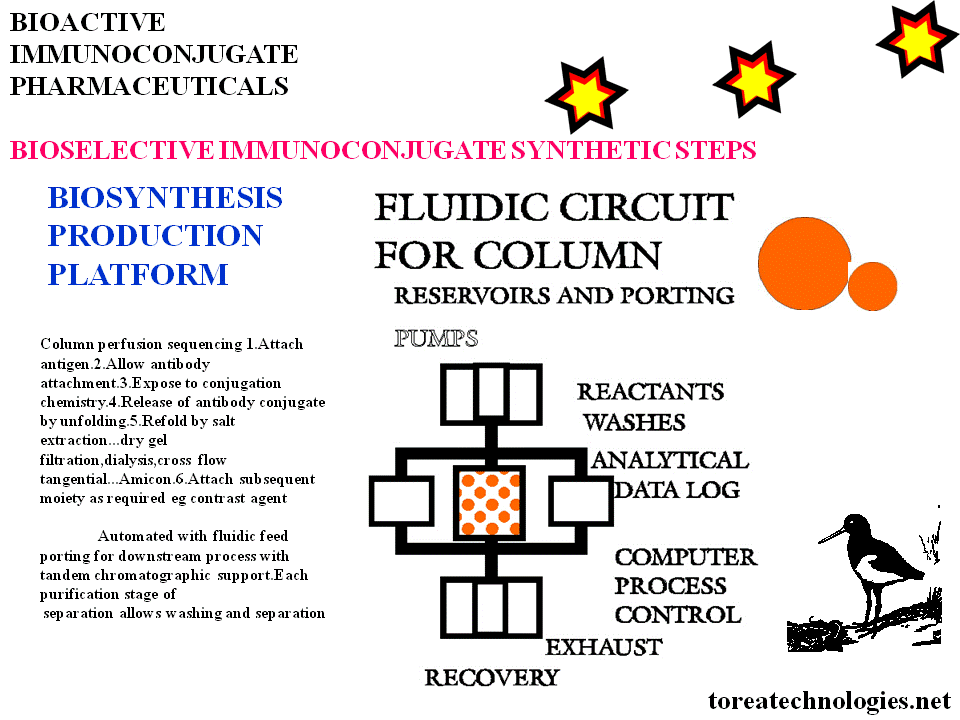
BIOSELECTIVE IMMUNOCONJUGATE SYNTHETIC STEPS
Column perfusion sequencing
1.Attach antigen.

2.Allow antibody attachment.

3.Expose to conjugation chemistry.
4.Release of antibody conjugate by unfolding.
5.Refold by salt extraction...dry gel filtration,dialysis,cross flow tangential...Amicon.
6.Attach subsequent moiety
as required eg contrast agent
Automated with fluidic feed porting for downstream process with tandem chromatographic support.
Each purification stage allows washing and separation
 IMMUNOREACTIVITY ASSAY
IMMUNOREACTIVITY ASSAY
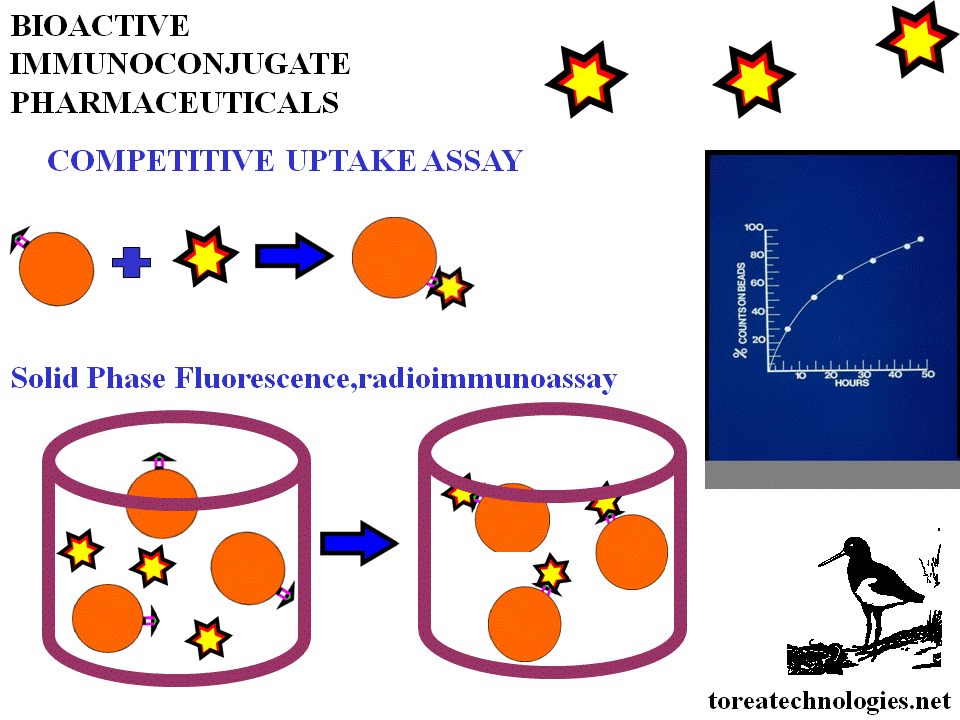
...............................................................................................
IMAGING
Advantages of bioselective scanning immunoconjugates
Diagnostic imaging contrast agents
SCINTIGRAPHY
MRI
Clinical high resolution imaging from enhanced bioactive targeting properties of agents with increased tumor uptake and lower background. Higher specific activity of agents whilst retaining immunoreactivity and binding. Earlier more accurate diagnosis and staging- quicker image acquisition time.
SCINTIGRAPHY
Clinically utilized in non-invasive screening -Hospital Nuclear Medicine Departments-gamma camera imaging
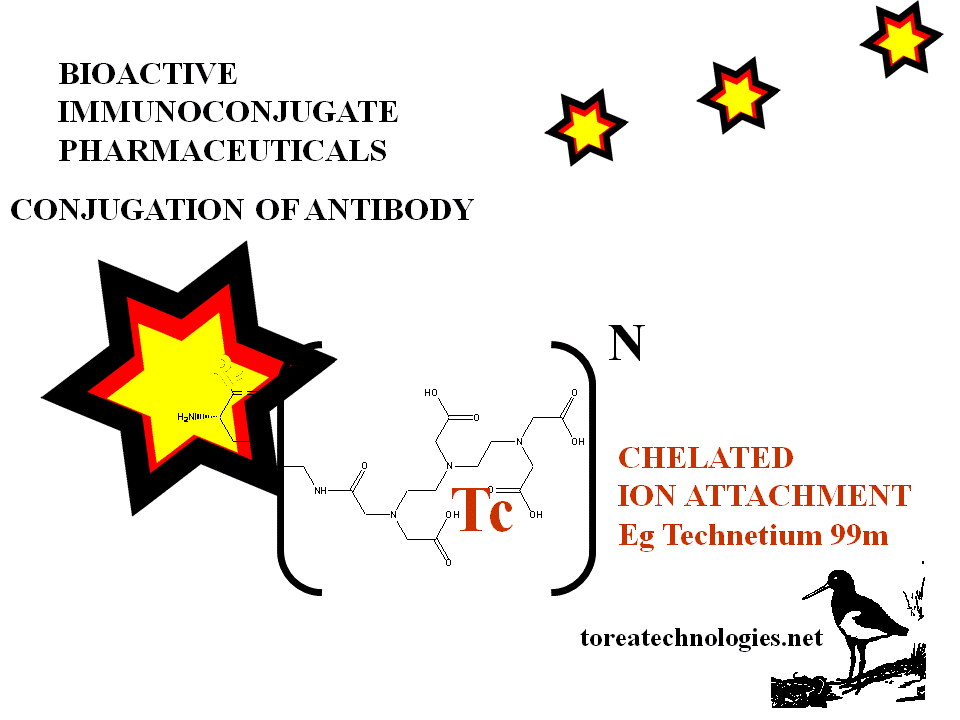
Alternative generic linked and chelated radionuclides Tc 99m I123 In111
Diverse antigenic targets eg fibrin,myosin and cancer
Higher substitution of bifunctional chelators and linkers eg.dtpa,dota,mag,bat, macrocycles and biotin.
Label attachment for activated antibody eg Tc99m from generator eg stannous reduction
Availability as kit ready to use from conjugation bioprocess
MRI
Magnetic resonance imaging
Gadolinium MRI scanning
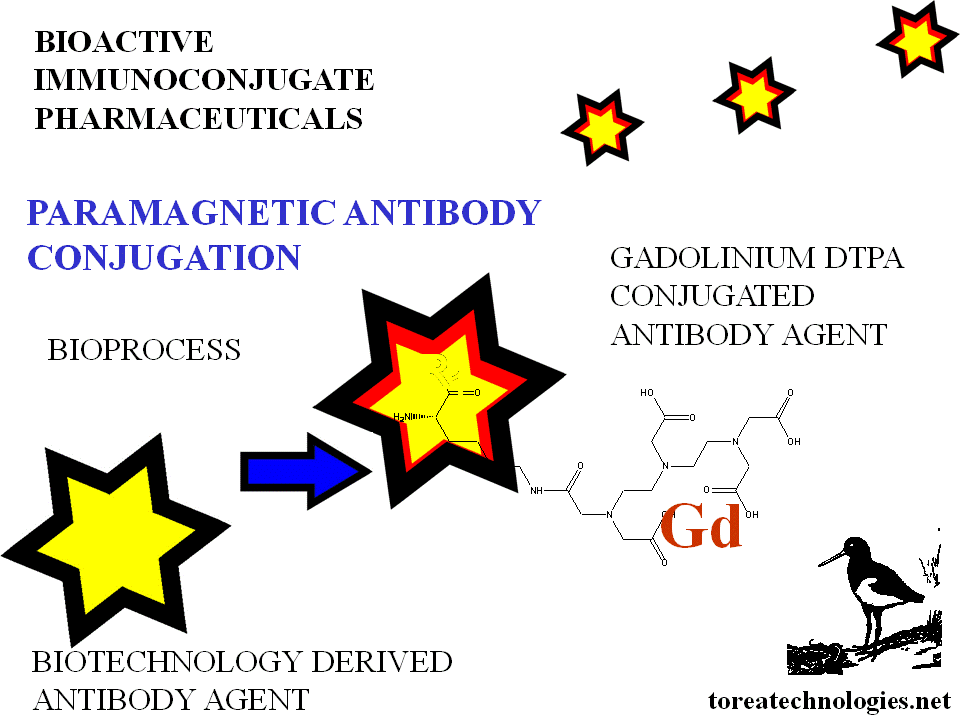
Magnetic contrast agents linked via bifunctional linkers eg gadopentate
Greater potency ensuring better tumor uptake increasing magnetic field strength at lesion site.
Alternative generic chelated paramagnetic agents becoming available eg Mn
Availability as kit ready to use from bioprocess
RADIOTHERAPY
Therapeutic Radionuclide Labels
Yttrium, Copper, Samarium via Chelated Linkers
eg. DTPA, DOTA, MAG, MACROCYCLES
Yttrium-90 Ibritumomab Zevalin is available commercially
Efficacy is able to be enhanced with bioactive attachment
Conjugated
eg: Iodine 125, 131 Via Bolton Hunter
Iodine-131 tositumomab Bexxar is available commercially
Efficacy is able to be enhanced with bioactive attachment
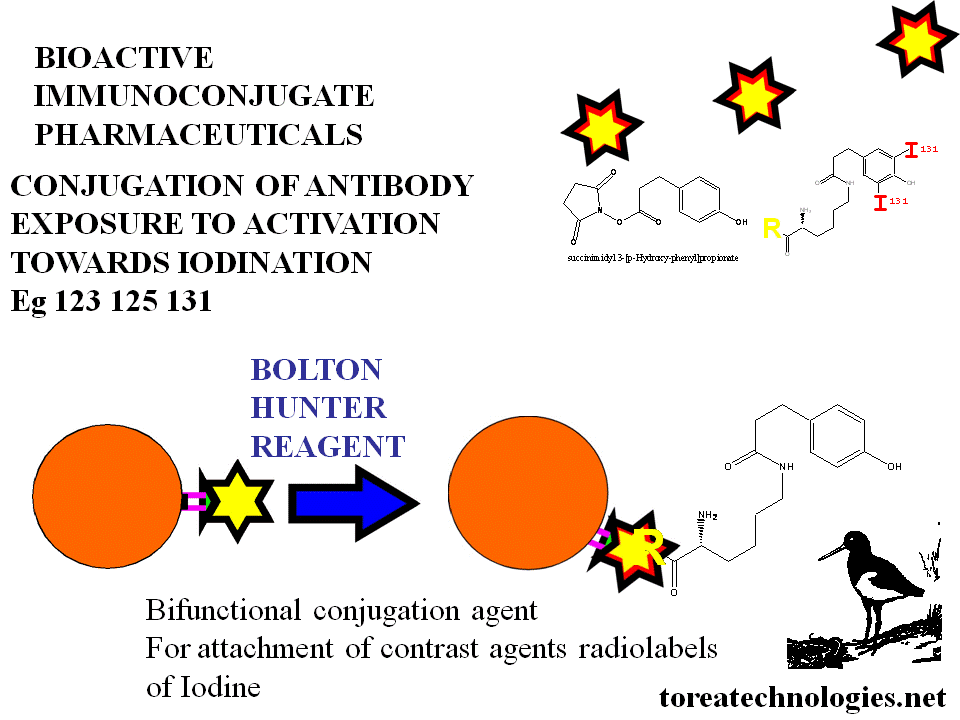
Attractive due to potency and small size for micrometasases, dissemination,conventional drug resistance and motile cancers
Alpha, Beta emitters with Dosimetry
.................................................................................


 Images
and
text copyright
Images
and
text copyright